
Photo by CDC on Unsplash
Neuralink’s Sight Implant Gets “Breakthrough Device” Tag from FDA
- Written by Andrea Miliani Former Tech News Expert
- Fact-Checked by Justyn Newman Former Lead Cybersecurity Editor
- Reader’s Comments 3
The United States Food and Drug Administration (FDA) gave Neuralink’s experimental implant to restore sight, Blindsight, the “breakthrough device” tag this Tuesday.
According to Reuters , this designation is provided to medical devices that can diagnose or treat severe medical conditions, and it’s intended to speed its development and review processes.
The update was shared on X by the neurotechnology company. “We have received Breakthrough Device Designation from the FDA for Blindsight,” states the post. “Join us in our quest to bring back sight to those who have lost it. Apply to our Patient Registry and openings on our career page.”
We have received Breakthrough Device Designation from the FDA for Blindsight. Join us in our quest to bring back sight to those who have lost it. Apply to our Patient Registry and openings on our career page https://t.co/abBMTdv7Rh — Neuralink (@neuralink) September 17, 2024
Elon Musk, co-founder of Neuralink, said on X that Blindsight is aimed to help those patients “who have lost both eyes and their optic nerve to see.” He explained that people who were born blind but still have a visual cortex could be able to see for the first time with the implant and mentioned that those who get the implant might see in low quality at first, but that within time their sight could be even better than natural vision and compared it to the fictional character from the show Star Trek, Geordi La Forge.
However, multiple news outlets have explained that Musk’s message is misleading and has also been considered “irresponsible” by the online news site Techcrunch . Journalist Devin Coldewey explained that the update does not imply that there is a cure for blindness, that it is still too premature to guarantee such results and Musk has publicly made big promises that might not be realistic.
Coldewey acknowledges that Neuralink has developed a powerful technology and that Blindsight can contribute to further medical advances.
While Musk has a reputation for making unfulfilled promises , Neuralink has made significant progress with its technology. The company recently installed another Link implant for the PRIME Study successfully on a second patient.
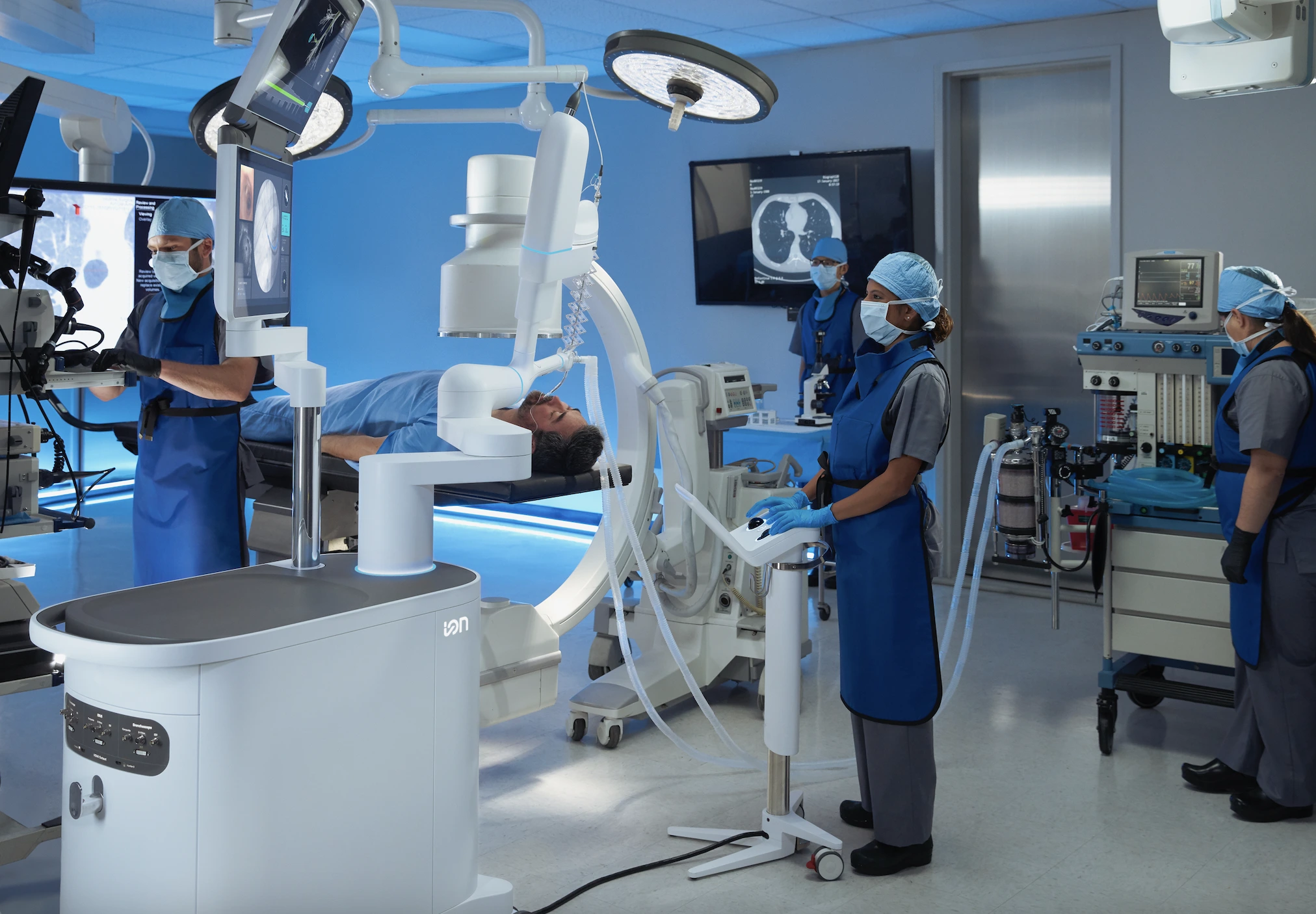
Image of Ion endoluminal system, from Intuitive Press Resources
“Game-Changing” Robot For Early Lung Cancer Detection Now Operational
- Written by Kiara Fabbri Former Tech News Writer
- Fact-Checked by Justyn Newman Former Lead Cybersecurity Editor
In a Rush? Here are the Quick Facts!
- New robotic tool promises quicker and more precise biopsies
- Builds on identifying over 600 early-stage lung cancer cases
- The system allows precise sampling from hard-to-reach lung areas
NHS Wythenshawe Hospital in Manchester has announced on Tuesday that it will introduce the Ion Endoluminal System. This new robotic tool is designed to improve early detection of lung cancer. It is expected to cut the time needed for diagnosis and treatment by several months.
The announcement states that the Ion Endoluminal System builds on the success of Wythenshawe’s Greater Manchester Lung Health Check programme, which has identified over 600 cases of lung cancer at early, treatable stages.
Diagnosing lung cancer and initiating treatment can be challenging because traditional biopsy methods often struggle to access small lung nodules, as noted in the announcement.
As a result, patients with suspected lung cancer may endure months of “watchful waiting,” during which nodules are monitored through scans until they are sufficiently large for biopsy.
The new Ion system addresses this issue with a thin robotic catheter that enables doctors to reach even the deepest and most difficult areas of the lungs with precision.
This system has already been used to perform biopsies on initial patients with suspected lung cancer, as reported on the announcement.
Dr. Haval Balata, Respiratory Physician and Clinical Lead for the Ion service at Manchester University NHS Foundation Trust, noted that this new technology could significantly improve patient care.
With the Ion system, patients may receive a diagnosis and start treatment or be cleared of cancer months earlier than with previous methods.
Dr. Haval Balata notes on the announcement, “Having this new innovative technology available for our patients to access is game changing.’’
Dr. Balata added, “ [The Ion system] allows us to offer patients the best possible treatments sooner rather than later, when treatment is much more likely to be successful.’’
Lung cancer is the leading cause of cancer-related deaths in the UK. Patients diagnosed at the earliest stage are nearly 20 times more likely to survive for five years compared to those diagnosed at a later stage, report the announcement.
Each year, approximately 48,000 people are diagnosed with lung cancer, and around 35,000 people die from the disease in the UK, as stated in the annoucement.